Analysis by Dr. Joseph Mercola
STORY AT-A-GLANCE
- Unbeknownst to the public, pork producers in the U.S. and Canada have been using customizable mRNA-based “vaccines” on their herds since 2018
- The mRNA platform, Sequivity, is the only part of this gene-based “vaccine” technology that has been approved. All customized mRNA injections created using this platform are untested, and the initial safety testing — upon which the assumed safety of all customized jabs are based — was grossly inadequate
- According to the U.S. Department of Agriculture’s (USDA) summary of studies supporting the product licensure of Sequivity, safety is based on a single study involving 748 piglets, which were given two doses of an unspecified mRNA Sequivity injection
- 525 piglets — 70.2% — experienced no adverse events. The remaining 29.8% suffered a serious adverse event, including 24 deaths. When adding together death, anorexia (wasting) and unthrifty (failure to thrive), 11.5% of the animals were lost to vaccine injury
- The USDA license approval for Sequivity is very weak, and only includes data for influenza, even though Merck is advertising vaccines for four other pathogens under the Sequivity line. Reading through Canada’s Environmental assessment also makes it clear that safety claims are theoretical only
As previously reported, unbeknownst to the public, pork producers in the U.S.1 and Canada2 have been using customizable mRNA-based “vaccines” on their herds since 2018. As it turns out, the mRNA platform, Sequivity,3,4 is the only part of this gene-based “vaccine” technology that has been approved.
All customized mRNA injections created using this platform are untested, and the initial safety testing — upon which the assumed safety of all customized jabs are based — was grossly inadequate. As noted by Zoetis, the largest producer of veterinary drugs and vaccines:5
“Sequivity has safety and efficacy studies based on the platform with a historical initial isolate, not likely the isolate that customers would be requesting in their product.”
We don’t even know what that initial isolate was. The Sequivity platform allows customized “vaccines” to be created as follows, in as little as eight weeks:6
- Pathogen is collected and sent to a diagnostic lab.
- The gene of interest is sequenced and sent electronically to Sequivity analysts.
- A synthetic version of the gene of interest is synthesized and inserted into the RNA production platform.
- The RNA particles released from incubated production cells are harvested and formulated into a customized “vaccine.”
USDA Lists Only One Safety Study for Sequivity
So, just what kind of safety testing has been done on these mRNA swine jabs? As it turns out, not much, and even saying that would be an exaggeration. Looking at the U.S. Department of Agriculture’s (USDA) summary of studies supporting the product licensure of Sequivity,7 we find only ONE safety study listed.
There are two efficacy studies pertaining to H1N1 swine influenza, two efficacy studies pertaining to H1N2 swine influenza, one efficacy study pertaining to H3N2 swine influenza, and one safety study pertaining to all vaccines under “typical use conditions.”
These six studies were all performed between June 2020 and December 2021. A search of the USDA’s Licensed Veterinary Biological Products catalog renders just this one summary of studies to support Sequivity’s licensure.8 This implies the platform was approved by the USDA based on these data alone.
While that’s concerning enough, it becomes even more questionable when you consider the side effects summary. The study involved a total of 748 pigs at three different testing sites, which were given two doses of an unspecified mRNA Sequivity injection three weeks apart. The piglets were monitored for side effects for 21 days after each injection, or until the side effect was resolved.
According to the USDA summary, 525 piglets — 70.2% — experienced no adverse events. However, among the remaining 29.8%, 7.4% became anorexic, 3.2% died, 2.7% became lame, 0.4% suffered anaphylaxis, 0.4% had some sort of central nervous system disorder, 0.3% developed meningitis and another 0.3% some form of musculoskeletal disorder. For the complete list, see the graph below, taken from page 18 of the USDA’s Summary of Studies.9
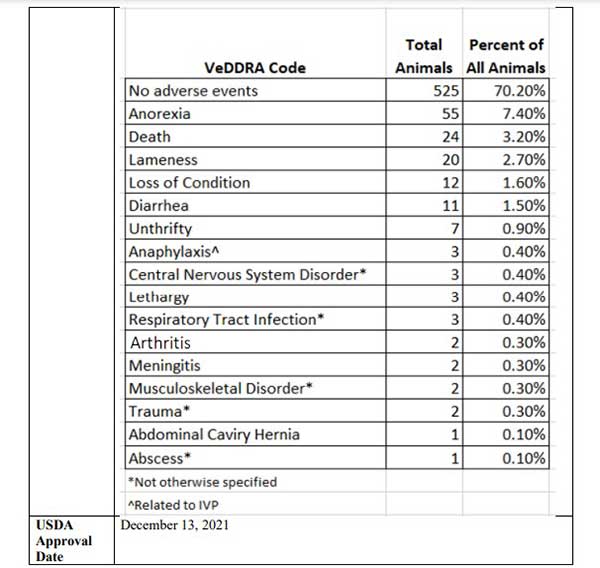
In all, 223 out of 748 piglets — nearly 30% — had some form of adverse event, including 24 deaths. How on earth is that even remotely considered safe? If 3 in 10 people suffered a serious side effect from a particular drug, and over 3% dropped dead immediately and 11.5% in the long run, would you consider it safe? Would you risk taking it? I sure wouldn’t.
Also consider this. Anorexia is basically considered a wasting disease. “Unthrifty” is similar in that it’s the failure to grow or develop normally due to disease. Either way, whether the piglet is wasting or failing to grow, it’s a loss for the farmer. Now, when we add together death, anorexia and unthrifty, you end up with 86 animals — 11.5%.
Losing 11.5% of your herd to vaccine death, wasting and failure to thrive really doesn’t make sense. That’s a loss of more than 1 out of 10 animals, which hardly speaks to either safety or effectiveness. Moreover, there are no safety studies at all related to human consumption of Sequivity-treated animals.
No Large-Scale Safety Studies Were Ever Intended
Interestingly, large-scale safety studies don’t appear to ever have been part of the plan. As noted in the Canadian government’s environmental assessment of Merck’s RNA particle prescription products for swine influenza (now known as Sequivity), posted in July 2018:10
“The prescription products will not undergo large scale field safety testing … Merck Animal Health’s commercial SIV vaccine based on the RNA replicon particle platform was field safety tested in approximately 900 commercial pigs at three geographically separate locations in the USA …
Much of what is known about Merck Animal Health’s RNA replicon particle platform has been extrapolated from studies performed to characterize the company’s commercial vaccine: Swine Influenza Vaccine, RNA (USDA Product Code 19A5.D0, CCVB File 880VV/S3.0/H16).”
USDA Product Code 19A5.D0 refers to the very first RNA-based “vaccine” for swine, developed by Harrisvaccines and licensed in 2012.11,12,13 Harrisvaccines was acquired by Merck Animal Health in 2015 and 19A5.D0 became theirs.14,15
The Canadian environmental assessment also notes that the mRNA is encased in a lipid envelope originating from the VERO African green monkey kidney cell line — a continuous cell line shown to have tumorigenic potential16 (i.e., may encourage cancer growth). It also reviews how the virus is genetically modified before the RNA transcripts are purified. So, as with the COVID jab, the mRNA is not identical to what’s found in the natural pathogen. It’s modified.

Download this Article Before it Disappears
Sequivity Is Gene Therapy
In 2012, Jodi French, a USDA Liaison at Harrisvaccines stated:17
“When we submitted our license application in 2009, the USDA didn’t have an established category into which the product fit. There were additional regulatory hoops to jump through in order to achieve this first license.”
In other words, the first mRNA-based injection for swine did not qualify as a vaccine. The same was true for the first mRNA-based shots for humans, the COVID-19 jab. The only definition it met — before the definition of “vaccine” was altered to make it fit18 — was gene therapy.19,20,21 I provided the details of this in “Why Is the Associated Press Lying About Gene Therapy Shots?”
When Sequivity was introduced in 2018, Merck also inadvertently delineated it as gene therapy, as they specifically defined the difference between real vaccines and the mRNA technology:22
“Vaccination mimics infection by introducing inactivated whole pathogens (antigens) into the animal to stimulate the immune system. For a conventional vaccine, the antigen is grown in the lab, deactivated or killed and then presented to the body. However, with SEQUIVITY and its revolutionary RNA Particle Technology, an electronic gene sequence is utilized.
After receiving the sequence, it is synthesized into RNA and inserted into the SEQUIVITY platform, which generates RNA particles. When injected in the animal, these particles provide instructions to the immune cells to translate the sequence into proteins which act as antigens.”
Transitioning to mRNA Jabs Has Likely Been the Plan for Years
Interestingly, in their June 2018 press release announcing the introduction of Sequivity, Merck described vaccination as the introduction of an antigen to “stimulate the immune system.” But that wasn’t the official definition of vaccination back then.
All the way up until the end of October 2021, a vaccine was defined as “a product that stimulates a person’s immune system to produce immunity to a specific disease, protecting the person from that disease.” Immunity, in turn, meant that “If you are immune to a disease, you can be exposed to it without becoming infected.”23
That definition didn’t change until the end of October 2021, when it suddenly became “A preparation that is used to stimulate the body’s immune response.”24 So, a “vaccine” went from being something that produces protective immunity, to simply stimulating an immune response.
Yet Merck was using this misleading language back in 2018 when discussing animal vaccines. To me, this suggests vaccine makers may have known, for several years, that mRNA-based “vaccines” are leaky and for whatever reason cannot prevent disease.
Were the mRNA COVID shots not tested to see if they prevented infection because they already had the answer, having used mRNA “vaccines” in animals for a couple of years already?
Are All mRNA ‘Vaccines’ Leaky?
If it’s true that the vaccine industry has known that mRNA “vaccines” can’t ever block infection, what does that tell us about the many human mRNA “vaccines” in the pipeline? Ultimately, they want to transition all vaccines to mRNA, yet it appears the mRNA platform has a major problem — it doesn’t appear to prevent disease.
On its Licensed Veterinary Biological Product Information page (last updated in February 2023),25 the USDA even admits that veterinary vaccines “may be considered effective without producing 100% protection against disease. Many products instead reduce the severity of disease.” This is precisely what we’re seeing with mRNA products, but that’s not the historical view of vaccines.
The veterinary side has apparently shifted to accept leaky vaccines as part of the norm, but there’s a significant danger to that, because vaccines that don’t effectively block infection drive mutations. Leaky vaccines were always considered problematic for this reason.
Of course, this doesn’t much matter if you’re cooking up customized mRNA shots in mere weeks, but this process may still push pathogens to mutate in all sorts of unpredictable directions. At the end of the day, it’s probably not a good idea to transition to a system where ALL vaccines are leaky and speed up mutations.
Evidence Supporting Sequivity Is Beyond Weak
In summary, the USDA license approval for Sequivity is very weak, and only includes data for influenza, even though Merck is advertising vaccines for four other pathogens under the Sequivity line.
Reading through Canada’s Environmental assessment also makes it clear that safety claims are theoretical only. Concerns are explained away without evidence in the form of animal testing, analysis of tissue or any type of direct safety testing for humans that eat this gene therapied pork.
What is certain is that people were not informed of this change in their food, and the meat industry is now caught in a difficult bind trying to explain why they swept safety under the rug and acted as though they’d never have to answer consumer questions.
Are mRNA-Jabbed Livestock Safe to Eat?
Considering health authorities insist the COVID shots are safe, it’s no wonder they also insist there are no problems associated with eating mRNA-treated meat. But can we trust them?
Livestock such as swine are routinely vaccinated against several diseases,26 and many of these vaccines must be administered at specific times to ensure there’s no residue left in the meat. When using the mRNA platform, however, there’s no time limit. So, just when are swine receiving these customized mRNA shots? And could there be mRNA in the pork you buy and eventually eat?
Vaccines are nearly always given in the hindquarter of the animal, and according to mRNA jab developers, the mRNA remains at the injection site. This theory has long since been proven false, as the mRNA in the COVID jab gets has been shown to be distributed throughout the human body.
But it makes sense that the mRNA might be more concentrated at the injection site. In livestock, this could be bad news, seeing how the hindquarters are usually where the prime cuts of meat come from. So, knowing whether there’s any mRNA left in the animal at the time of slaughter is important.
We also need to understand how long the antigen produced by the animal’s cells in response to a customized mRNA shot sticks around, and whether ingesting that antigen might have repercussions for human health. If veterinary mRNA shots are anything like the COVID jab, we could potentially be in trouble.
As reported by Jessica Rose, a postdoctoral researcher in biology, Pfizer’s and Moderna’s bivalent COVID jabs are grossly contaminated. Both have been found to contain 20% to 35% expression vectors — double-stranded DNAs used in the manufacturing of the mRNA — that are transformation-competent in E. coli. She writes:27
“Take home message: The left-over expression vectors used to manufacture the mRNAs are at contamination levels 100-fold higher than originally proposed and imply trillions of DNA molecules per dose. This has implications for integration into our genome.”
For a scientific deep dive into how and why this kind of contamination has implications for genome integration, read through Rose’s article, and/or Anandamide’s Substack article28 covering the same topic. The point I want to make is this: Might there be leftover expression vectors in the animal vaccines as well? And if so, can they integrate into the animal’s genome — and/or into ours when we eat their meat?
Big Ag Relies on Big Pharma Shills to Dismiss Concerns
The fact that the meat industry has no solid science to back its safety claims is evident by the fact that they’re relying on long-time Big Pharma shills like Dr. Kevin Folta to diffuse and “pooh-pooh” consumer concerns.
Folta, a University of Florida professor, is a longtime advocate for genetically modified organisms (GMOs). He has also advocated for the safety of glyphosate, and in 2015, he was caught lying about his financial ties to Monsanto.
Now, he’s taken up the advocacy for mRNA shots in livestock. Cowboy State Daily interviewed him for an April 5, 2023, piece29 that sought to dismiss concerns about gene therapied pork. Two weeks later, Folta showed up in an article on porkbusiness.com.30
“There is no integration into the DNA,” Folta told Cowboy State Daily. “It’s a transient set of instructions, like a USB drive. Not a hard drive. Messenger RNA occurs naturally as part of the function of cells in the body. mRNA is everywhere, and you can’t live without mRNA. It’s very temporary. So, when an animal is slaughtered or when a plant dies, mRNA is the first thing to go.”
Folta made even a bigger fool of himself in his interview with porkbusiness.com, claiming the “mRNA never leaves the cells from where it was injected.” Many of you will know exactly what’s wrong with Folta’s arguments that mRNA is “everywhere” and therefore harmless, that its activity is temporary because in its native form, it’s so fragile and perishable, and that it all stays in the injection site. None of Folta’s argument is true.
The mRNA in the shots is synthetic and does NOT break down the way normal mRNA does. This is an indisputable scientific fact, so he is clearly misleading people. Everyone who knows even the slightest bit about this mRNA jab technology knows the synthetic mRNA has been designed to prevent rapid breakdown and is further stabilized by the nanolipid.
Pfizer’s own research has also conclusively proven the mRNA spreads throughout the body. It most certainly does not stay in the cells around the injection site. So, Folta’s arguments are beyond irrational and don’t hold any water. They don’t even make l sense superficially.
mRNA Enters State Legislation
Missouri is now tackling this food safety issue head-on with House Bill 1169.31,32 If passed, it would require labeling of products that can alter your genes. It also asserts that fully informed consent must be given for all vaccines, gene therapies and medical interventions, and would require companies to share information about the potential transmissibility of gene-altering interventions.
The pushback by industry against this bill, which only calls for transparency, has been enormous. Transparency is, apparently, a serious threat to industry, and that means it would most likely be empowering for consumers. Consumer rejection of gene therapied meats could not only destroy Big Ag. It could also hamstring Big Pharma’s attempts to use the food supply as a tool to distribute vaccines unbeknownst to consumers.
If this bill is passed in Missouri, it could help protect the food supply of the entire United States. In the meantime, I recommend avoiding all pork products, including organic ones, as they not only have high levels of the omega-6 fat, linoleic acid, due to the grains they are fed, but virtually all are likely contaminated with mRNA “vaccines.”
- 1 YouTube Global Ag Media 2018
- 2, 10 Government of Canada July 24, 2018
- 3, 6 Merck Animal Health, Sequivity
- 4 Merck Sequivity White Paper
- 5 ZoetisUS.com Product Comparison Chart
- 7, 9 USDA Summary of Studies Supporting USDA Product Licensure, P. 17-18
- 8 USDA APHIS Search for Sequivity
- 11, 17 PR Newswire September 20, 2012
- 12 Watt Poultry October 2, 2012
- 13 Drugs.com RNA Swine Influenza Vaccine
- 14 Merck November 12, 2015
- 15 Merck March 15, 2016
- 16 J Biol Stand October 1984; 12(4): 391-398
- 18, 23 Pulse Stubstack November 9, 2021
- 19 Moderna’s SEC Form S-1 Registration Statement
- 20 US SEC BioNTech 2019
- 21 FDA Guidance for Industry January 2020
- 22 Merck June 6, 2018
- 24 CDC Immunizations: The Basics, Definition of Terms
- 25 APHIS USDA Licensed Veterinary Biological Product Information as of February 2, 2023, third bullet point
- 26 Swine Vaccine Protocol
- 27 Jessica Rose Substack March 9, 2023
- 28 Anandamide Substack March 8, 2023
- 29 Cowboy State Daily April 5, 2023
- 30 Pork Business April 19, 2023
- 31 Missouri House bill 1169
- 32 Tom Renz Substack April 2, 2023





















Thanks! Share it with your friends!
Tweet
Share
Pin It
LinkedIn
Google+
Reddit
Tumblr