Analysis by Dr. Joseph Mercola
STORY AT-A-GLANCE
- According to Brook Jackson, a whistleblower who worked on Pfizer’s Phase 3 COVID jab trial, data were falsified, patients were unblinded, the company hired poorly trained people to administer the injections, and follow-up on reported side effects lagged way behind
- The FDA did not follow up on Jackson’s complaint or investigate the allegations before granting full licensing to Pfizer’s Comirnaty shot
- FDA now wants 75 years to drip out the data it relied on to grant full licensing to Comirnaty
- An adverse event report from Pfizer, covering December 2020 through the end of February 2021, shows the shot causes severe and often long-term, unresolved injuries
- Pfizer’s data also show the shot causes severe injuries in pregnant and nursing women. Based on these data alone, which the FDA was aware of at the end of April 2021, the Pfizer shot should have been pulled from the market
According to Brook Jackson, a whistleblower who worked on Pfizer’s Phase 3 COVID jab trial in the fall of 2020, data were falsified, patients were unblinded, the company hired poorly trained people to administer the injections, and follow-up on reported side effects was significantly delayed.
Her testimony was published November 2, 2021, in The British Medical Journal by investigative journalist Paul Thacker, who noted that:1
“[F]or researchers who were testing Pfizer’s vaccine at several sites in Texas during that autumn, speed may have come at the cost of data integrity and patient safety … Staff who conducted quality control checks were overwhelmed by the volume of problems they were finding.”
December 2, 2021, The Last American Vagabond interviewed Jackson (video above2) about what she saw while working on Pfizer’s trial. Jackson is a trained clinical trial auditor with more than 15 years’ experience in clinical research coordination and management.
She had previously held a director of operations position before she was hired in early September 2020 by the Ventavia Research Group, a research organization charged with testing Pfizer’s COVID jab at several sites in Texas. Right from the start, Jackson was struck by the chaotic nature of the operation.
She also felt the informed consent was inadequate, considering the novel nature of the mRNA gene transfer technology. On top of that, she found the crash cart contained expired medications, and some important emergency medications — were a participant to suffer an acute adverse event — were missing entirely.
Data Forgery Among the Many Problems Identified
Jackson claims she repeatedly informed her superiors of poor laboratory management, patient safety concerns and data integrity issues. When she realized her concerns were ignored, she finally filed a complaint with the U.S. Food and Drug Administration. In her complaint to the FDA, Jackson listed a dozen incidents of concern, including the following:
- Participants were not monitored by clinical staff after receiving the shot
- Patients who experienced adverse effects were not promptly evaluated and protocol deviations were not being reported
- The Pfizer injection vials were stored at improper temperatures
- Laboratory specimens were mislabeled
Later that same day, Jackson was fired. According to her separation letter, management decided she was “not a good fit” for the company. According to Jackson, this was the first time she’d ever been fired in her 20-year career as a clinical research coordinator. As noted by Thacker:3
“In a recording of a meeting in late September 2020 between Jackson and two directors a Ventavia executive can be heard explaining that the company wasn’t able to quantify the types and number of errors they were finding when examining the trial paperwork for quality control. ‘In my mind, it’s something new every day,’ a Ventavia executive says. ‘We know that it’s significant.’
Ventavia was not keeping up with data entry queries, shows an email sent by ICON, the contract research organization with which Pfizer partnered on the trial. ICON reminded Ventavia in a September 2020 email: ‘The expectation for this study is that all queries are addressed within 24hrs.’
ICON then highlighted over 100 outstanding queries older than three days in yellow. Examples included two individuals for which ‘Subject has reported with Severe symptoms/reactions … Per protocol, subjects experiencing Grade 3 local reactions should be contacted. Please confirm if an UNPLANNED CONTACT was made and update the corresponding form as appropriate.’
According to the trial protocol a telephone contact should have occurred ‘to ascertain further details and determine whether a site visit is clinically indicated.’ Documents show that problems had been going on for weeks.
In a list of ‘action items’ circulated among Ventavia leaders in early August 2020, shortly after the trial began and before Jackson’s hiring, a Ventavia executive identified three site staff members with whom to ‘Go over e-diary issue/falsifying data, etc.’ One of them was ‘verbally counseled for changing data and not noting late entry,’ a note indicates.”
Jackson’s disclosures were recently featured in the Italian documentary, “Pfizergate.”4,5 The documentation she gathered are available for download on the COVID Vaccine Reaction’s website.6
Ventavia, Pfizer and FDA Ignore Accusations
Strangely enough, the extent of Ventavia’s effort to defend itself has been to deny that Jackson ever worked on the Pfizer trial — a charge that is verifiably false, as she has documentation proving she was assigned to work on the trial.7
Pfizer has also remained mum on the issue. The company did not reply to any of The BMJ’s questions, one of which was whether Ventavia’s data were incorporated into Pfizer’s safety and efficacy analyses.
We do know, however, that none of the problems Jackson raised in her complaint to the FDA were noted or addressed in Pfizer’s briefing document, submitted to the FDA’s advisory committee meeting December 20, 2020, when its emergency use authorization application was reviewed.
The FDA went ahead and gave the Pfizer jab emergency use authorization the very next day, despite being in receipt of Jackson’s complaint, which ought to have put the brakes on the FDA’s authorization. At bare minimum, they should have investigated the matter before proceeding.
The BMJ has tried to get answers from the FDA as to why it has not inspected any of Ventavia’s trial sites in the wake of Jackson’s accusations, and whether other complaints about the trial have been received. An FDA spokesperson told The BMJ the agency cannot comment as it is “an ongoing matter,” whatever that means.
The FDA did say, though, that it has “full confidence in the data that were used to support the Pfizer-BioNTech COVID-19 Vaccine authorization and the Comirnaty approval.” Considering they’ve not investigated Jackson’s complaints, their vote of confidence doesn’t strike me as particularly convincing.
Other Ventavia Witnesses Speak Out
Jackson wasn’t the only employee to get sacked from Ventavia after raising concerns about the integrity of the Pfizer trial. According to Thacker, several other Ventavia employees either left or were fired. Among them is a Ventavia official who had participated in the late September meeting cited above. Thacker writes:8
“In a text message sent [to Jackson] in June the former official apologized, saying that ‘everything that you complained about was spot on.’ Two former Ventavia employees spoke to The BMJ anonymously for fear of reprisal and loss of job prospects in the tightly knit research community. Both confirmed broad aspects of Jackson’s complaint.
One said that she had worked on over four dozen clinical trials in her career, including many large trials, but had never experienced such a ‘helter skelter’ work environment as with Ventavia on Pfizer’s trial.
‘I’ve never had to do what they were asking me to do, ever,’ she told The BMJ. ‘It just seemed like something a little different from normal — the things that were allowed and expected.’”
According to these whistleblowers, problems persisted after Jackson’s firing. One of them claims there were, on several occasions, not enough staff to test trial participants who reported COVID-like symptoms.
Laboratory confirmed symptomatic COVID-19 was the primary endpoint of the trial, so this was a crucial task. An FDA review memorandum from August 2021 states that 477 trial participants with suspected COVID-19 were not tested for infection. “I don’t think it was good clean data,” the former Ventavia employee told Thacker. “It’s a crazy mess.”
Such statements clearly fly in the face of statements made by world leaders, health authorities and the mainstream media. Most, like federal health minister for Australia, Greg Hunt, have claimed the COVID shots have undergone “rigorous, independent testing” to ensure they’re “safe, effective and manufactured to a high standard.”9
Nothing we know so far supports such a conclusion. The testing has been far from rigorous and has not been independently verified.
Vaccine Adverse Events Reporting System (VAERS) data show they’re shockingly far from safe; real-world data show effectiveness wanes within a handful of months while leaving you more susceptible to SARS-CoV-2 variants and other infections; and manufacturing standards have also been shown lacking, as a variety of foreign contaminants have been found in the vials.10
Science Depends on Rigorous Data Collection
The video above is a short extract from a November 2, 2021, meeting organized by Sen. Ron Johnson, during which Peter Doshi, Ph.D., associate editor of The BMJ, reviewed some of the many concerns experts have about the integrity of the COVID jab data.
He pointed out that Pfizer’s raw trial data will not be made available until May 2025. So far, Pfizer has refused to release any of its raw data to independent investigators and, without that, there’s no possible way to confirm that what Pfizer is claiming is actually true and correct.
In other words, we’re expected to simply take the word of a company that has earned a top spot on the list of white collar criminals; a company that in 2009 was fined a record-breaking $2.3 billion in fines for fraudulent marketing and health care fraud.11 Press releases are not science. They’re marketing. Without the raw data, we have no science upon which to base our decisions about the COVID kill shot.
Doshi stressed how utterly unscientific a process we’re now following. He also points out that doctors have an ethical duty to not recommend a treatment for which they have no data. Quoting from a 2020 article he co-wrote:12
“Data transparency is not a ‘nice to have.’ Claims made without access to the data — whether appearing in peer reviewed publications or in preprints without peer review — are not scientific claims.
Products can be marketed without access to the data, but doctors and professional societies should publicly state that, without complete data transparency, they will refuse to endorse COVID-19 products as being based on science.”
“The point I am trying to make is very simple,” Doshi said. “The data from COVID vaccines are not available and won’t be available for years. Yet, we are not just ‘asking’ but ‘mandating’ millions of people to take these vaccines … Without data, it’s not science.”
FDA Wants 75 Years to Release Pfizer Trial Data
In September 2021, a group called Public Health and Medical Professionals for Transparency (PHMPT) filed a Freedom of Information Act (FOIA) request with the FDA to obtain the documentation used to approve Comirnaty, including safety and effectiveness data, adverse reaction reports and lists of active and inactive ingredients.
In their FOIA application, the PHMPT asked the agency to expedite release of the documents — a reasonable request, considering we have no raw data and the shots are being pushed on children as young as 5. When, after a month, the FDA still had not responded to the FOIA request, the PHMPT sued.13
The FDA initially asked the judge to allow them to delay the full release of all documents — a total of 329,000 pages — until 2076, doling out just 500 pages per month. The judge agreed.
A short while later, the FDA claimed it found another 59,000 pages, which would necessitate tacking on another 20 years.14 The full release, according to the FDA, can’t be completed until 2096, at which time most of us will be dead and buried. As noted by Aaron Siri, the lawyer working on the case on behalf of the PHMPT:15
“If you find what you are reading difficult to believe — that is because it is dystopian for the government to give Pfizer billions, mandate Americans to take its product, prohibit Americans from suing for harms, but yet refuse to let Americans see the data underlying its licensure.”
All of that said, the initial release of some 92 pages are so damning, we won’t need hundreds of thousands of pages to make an assessment as to the safety of these shots. In fact, the data are so incredibly bad, it raises serious questions about how the FDA could possibly conclude that the Pfizer shot is safe enough to use, especially on pregnant women and children.
Shocking Revelations in First Batch of FOIA Docs
In mid-November 2021, two months after the lawsuit against it was filed, the FDA released the first batch of 91 pages,16,17 which reveal the FDA has been aware of shocking safety issues since April 30, 2021.
Cumulatively, through February 28, 2021, Pfizer received 42,086 adverse event reports, including 1,223 deaths, primarily from the U.S., U.K., Italy, Germany, France and Portugal. Of those adverse events, 25,379 were medically confirmed. Below is a chart from one of the documents,18 showing a general overview of the reported outcomes.
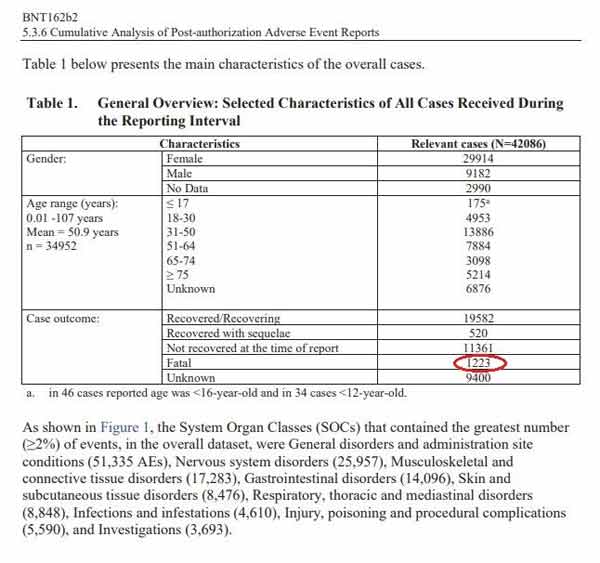
To have 1,223 fatalities and 42,086 reports of injury in the first three months is a significant safety signal, especially when you consider that the 1976 swine flu vaccine was pulled after only 25 deaths.
In the video above, Melanie Risdon with the Western Standard interviews Dr. Daniel Nagase, a doctor in Alberta, Canada, who was stripped of his Alberta medical license after successfully treating COVID-19 patients with ivermectin. Nagase reviews other equally devastating data in these documents.
He points out that of the 42,086 patients who were injured at some point during those first three months, 520 of them were diagnosed with a long-term disability or condition as a result. Not recovered at the time of the report were 11,361. That means 27% of those injured had not recovered from their adverse event.
When you add it all together: the 1,223 deaths, the 520 long-term disabilities and the 11,361 who had not recovered from their injury, you end up with just over 31%.
In other words, nearly 1 in 3 people who got the shot and suffered an adverse effect ended up dead, permanently disabled or with long-term unresolved injury. “This should be front-page news,” Nagase says. How can the FDA look at this and conclude that the shot is safe? Clearly, when people get injured by this shot, they’re often injured very badly.
Pfizer Data Prove Shot Is Unsafe for Pregnant Women
On page 12 of the “Cumulative Analysis of Post-Authorization Adverse Event Reports Received Through 28-Feb-2021” document,19 you find data on pregnant and lactating women. Here too, the results are hair-raising and should have triggered a complete stop to the injection campaign of pregnant and nursing women.
Disturbingly, they did not collect comprehensive data on these women, such as which trimester they were in when they received the shots. This again points to serious problems with Pfizer’s trial data collection. How do you include pregnant women in a trial and don’t collect basic information such as how many weeks pregnant they are?
On page 12 we find that out of 124 adverse event cases involving a pregnant woman, only 49 were non-serious and 75 were serious. So, out of the 274 pregnant mothers who reported an adverse event, 27% suffered a SERIOUS adverse event, such as a miscarriage or stillbirth. “That’s an incredible danger!” Nagase says and, again, the FDA has been aware of this danger since April 30, 2021.
The data also show there’s danger for breastfeeding mothers. Of the 133 nursing mothers who filed a report, 17 of the breastfed babies — 13% — suffered an adverse event through this secondary exposure (breastmilk), a finding that Nagase calls “absolutely stupendous.”
“So, this idea that the ‘vaccine’ sheds and transfers through breastmilk is absolutely true,” he says. “It’s proven by Pfizer’s own adverse event data.”
Children at Risk for Serious Long-Term Injury
Pfizer also received 34 adverse event reports involving children under the age of 12, the youngest being 2 months old. Of those, 24 were categorized as “serious” and only 10 were “non-serious.” So, of the children who were injured, 70.6% suffered SERIOUS injury.
How can our health agencies approve this COVID shot for children under the age of 12 when a vast majority of injuries, when they occur, are serious ones? What’s more, 13 of the children who were seriously injured remained unresolved as of February 28, 2021.
According to Nagase, based on these documents alone, Pfizer’s COVID shot should have been permanently pulled from the market. The reason it wasn’t, he believes, is because the medical and regulatory systems have both been corrupted and usurped by the drug industry. They want to make money off these shots, and our health authorities are covering up proven harms in order to facilitate profitmaking.
At the end of the day, only you can decide what’s in your best interest. But please, do review the actual science before you make your decision and don’t blindly trust corporate press releases and unsupported statements of safety.
Pfizer’s own data prove it’s not safe by any reasonable definition of the word, and that’s on top of the testimony of Jackson and others who have seen just how shoddy the data gathering is.
- 1, 3, 8 The BMJ 2021; 375:n2635
- 2 The Last American Vagabond December 2, 2021
- 4 Facebook Pfizergate
- 5 Ilsussidario.net Pfizergate
- 6 Covidvaccinereaction.com
- 7 The BMJ 2021;375:n2635 Rapid Response
- 9 Twitter Greg Hunt February 15, 2021
- 10 CNBC August 30, 2021
- 11 ABC Australia September 4, 2009
- 12 The BMJ 2020; 370; m3260
- 13 The Defender November 19, 2021
- 14, 15 Newsmax December 8, 2021
- 16 PHMPT.org Pfizer documents
- 17 thekylebecker.substack.com November 21, 2021
- 18, 19 Cumulative Analysis of Post-Authorization Adverse Event Reports Received Through 28-Feb-2021

















Thanks! Share it with your friends!
Tweet
Share
Pin It
LinkedIn
Google+
Reddit
Tumblr