Analysis by Dr. Robert Malone
STORY AT-A-GLANCE
- Taken together, this work clearly demonstrates that vaccine-induced protective antibody responses following a second and third dose of BNT162b2 are both very low and transient in older people
- The authors conclude that “additional booster doses may be necessary, particularly in older people.” … One has to wonder, whether the study authors really mean to imply that a total of 13 boosters per year are needed (52 weeks/four weeks = 13 boosters)? Or are they just writing this to get through peer review?
- A more unbiased conclusion based on these data is that the Pfizer/BioNTech BNT162b2 vaccine has exceptionally poor durability of neutralizing antibody response (using a non-validated or “academic research” level test) against currently circulating viral strains
- The failure to address these data clearly demonstrates, once again, both the failure of the peer review process, the irrational pro-vaccine bias of the authors, and the pro-vaccine bias of the editors of the Journal of the American Medical Association
This article was originally published here.
This 25 minutes video explains in detail the mechanisms of action for mRNA COVID-19 vaccines, as well as highlighting some of the known risks. If you wish to have a better understanding of the technology or wish for a refresher on the tech, as well as to learn a bit of cellular and molecular biology – this is a great primer. So, sit back, relax and open your mind to a bit of science.
Analysis of the Peer Reviewed Study
Neutralizing Antibodies Against the SARS-CoV-2 Omicron Variant (BA.1) 1 to 18 Weeks After the Second and Third Doses of the BNT162b2 mRNA Vaccine. JAMA Netw Open. 2022;5(5):e2212073. doi:10.1001/jamanetworkopen.2022.12073
The abstract of the paper first mentions that in early clinical trials, SARS-CoV-2 neutralizing antibodies were correlated with protection against infection and disease caused by early viral variants, but not mentioned by the authors – this correlation has not been demonstrated with the various Omicron variants.
The authors of this current work, which focuses on the Pfizer/BioNTech vaccine, note that in studies carried out by Moderna, there is an observed decrease in neutralizing antibody titers in the vaccinated population which corresponds to a decrease in vaccine efficacy against polymerase chain reaction – confirmed Omicron infection in Denmark and symptomatic Omicron infection in the United Kingdom.
The study has a curious selection bias – it only includes males, and it neither captures nor analyzes any data concerning efficacy or effectiveness endpoints.
This study repeatedly asserts that there is a demonstrated correlation between neutralizing antibody titers and protection against infection and disease, but relies on historic data from non-Omicron variants to support this claim.
- This study detected a rapid decline in Omicron-specific serum neutralizing antibody titers only a few weeks after the second and third doses of BNT162b2.
- For those aged 65 and older, there were almost no Omicron-specific serum neutralizing antibody titers after dose 2 (see Figure 2, panel B).
- For those aged 65 and older, there were almost no Omicron-specific serum neutralizing antibody titers by week 8 after dose 3.
Please review Figure 2, which clearly shows these results:
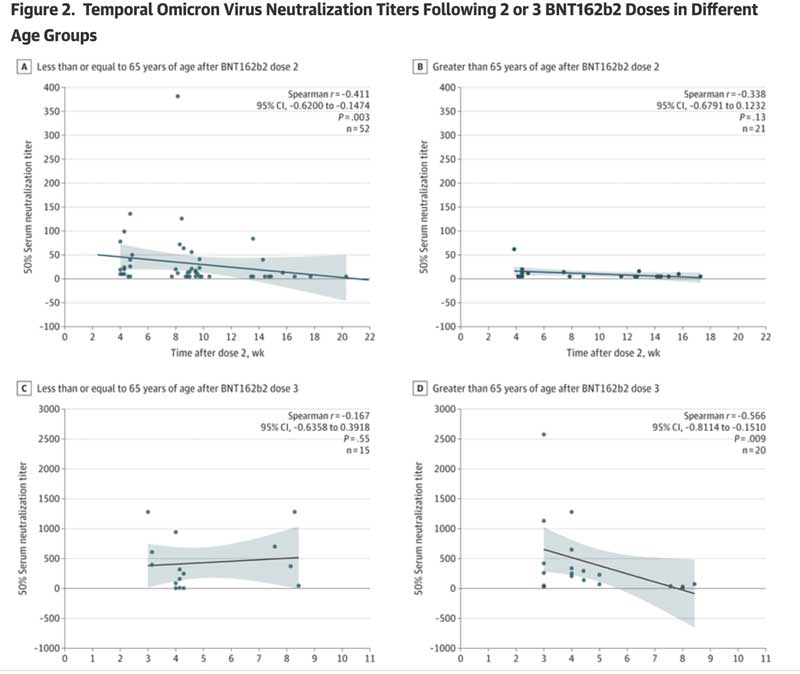
Taken together, this work clearly demonstrates that vaccine-induced protective antibody responses following a second and third dose of BNT162b2 are both very low and transient in older people (which is the cohort at most significant risk of hospitalization and death).
The article mentions that conserved T-cell immunity and non-neutralizing antibodies may still provide protection against hospitalization and death even as neutralizing antibodies wane, but the study did not actually measure T-cell immunity and non-neutralizing antibodies.
The authors conclude that “additional booster doses may be necessary, particularly in older people.” Following their logic, one can view the above table and note that the ranges of protection rapidly wanes after week 4 in those older than 65 years after dose 3. One has to wonder, whether the study authors really mean to imply that a total of 13 boosters per year are needed (52 weeks/four weeks = 13 boosters)? Or are they just writing this to get through peer review?
Also relevant is that “validated correlate of protection” is a precise regulatory term. It means that extensive analysis and characterization of both the specific test used (neutralizing antibodies against SARS-CoV-2), as well as the connection between what it is testing and the thing that it is being developed to serve as a surrogate for (protection) has been performed. In the case of the current study, neither has occurred.
Therefore, no conclusions can be drawn from this study concerning what it is measuring (purported to be neutralizing antibodies) and the endpoint for which it is to serve as a surrogate for (protection) can be drawn. In other words, this paper is essentially a preliminary study of no real regulatory significance — it has not been performed with the necessary rigor.
A more unbiased conclusion based on these data is that the Pfizer/BioNTech BNT162b2 vaccine has exceptionally poor durability of neutralizing antibody response (using a non-validated or “academic research” level test) against currently circulating viral strains.
In contrast, emerging mRNA vaccine effectiveness data from multiple countries are repeatedly demonstrating a dose dependent negative effectiveness of these vaccines (in other words, the more doses administered, the higher the risk of significant COVID disease or death).
The failure to address these data clearly demonstrates, once again, both the failure of the peer review process, the irrational pro-vaccine bias of the authors, and the pro-vaccine bias of the editors of the Journal of the American Medical Association.
When will objective, data-driven physicians and medical scientists ever be able to restore their prior faith in the Journal of the American Medical Association, or will JAMA continue to remain (for all practical purposes) just a pharmaceutical industry promotional trade journal?
















Thanks! Share it with your friends!
Tweet
Share
Pin It
LinkedIn
Google+
Reddit
Tumblr