Analysis by A Midwestern Doctor
STORY AT-A-GLANCE
- The SSRI antidepressants are some of the most harmful (but most profitable) medications on the market, and this was shown throughout their clinical trials
- In turn, once they hit the market, the FDA was overwhelmed with a deluge of adverse reactions being reported and the public demanding the drugs be investigated
- Remarkably, rather than address these issues, the FDA for decades did everything it could to cover up what was happening and protect the SSRIs
- The pharmaceutical executive who got Prozac (the first SSRI) to market testified that this required a variety of criminal tactics (e.g., overt bribery). Likewise, the Bush family was in bed with Prozac’s manufacturer and stocked their administrations with executives from the company, which further helps to explain the FDA’s unscrupulous conduct
In yesterday’s article, I reviewed the immensely concerning data that emerged throughout the SSRI antidepressant trials. Sadly, rather than this data being listened to, it was given a pass by the FDA, a pattern we have tragically seen occur with numerous highly lucrative pharmaceuticals. In my eyes, three things stand out about the SSRI saga.
The first is that numerous whistleblowers came forward and provided clear proof of exactly how this corruption transpired. The second is that the corruption reached the highest levels of government.
The third is that the FDA went to incredible lengths to protect the SSRIs, something many of us would not believe could be possible had we not just witnessed it throughout COVID-19.
Note: One of the greatest issues with the SSRIs is how addictive the drugs are (stopping them can cause severe withdrawals which are highly damaging to the nervous system and sometimes precipitate violent psychosis). If you are considering stopping them, I strongly recommend working with a health professional who is experienced in this regard.
For those who do not have access to one, I compiled a detailed summary of how to safely withdraw from them here (in the second half of this article).
John Virapen
It is exceedingly rare for a pharmaceutical executive to speak out against their industry (as doing so will permanently blacklist them from being hired again). In turn, the only ones I know of (besides an executive I’ve privately corresponded with) are Peter Rost and John Virapen, both of whom found themselves in very unique circumstances which enabled and compelled them to speak out against their industry and disclose the sociopathic behavior they observed within it.
Note: Rost’s story, along with similar accounts from the other Pfizer whistleblowers can be found in this article and this article.
One of the pharmaceutical executives directly involved in obtaining the approval for the original SSRI antidepressant, Prozac, developed a great deal of guilt for what he was complicit in once a large number of SSRI-linked deaths occurred. In turn, after he was unjustly fired, John Virapen chose to speak out.
Virapen chronicled those events in “Side Effects: Death — Confessions of a Pharma Insider.” These included outrageous acts of bribery to get his drugs approved, and photographing physicians with prostitutes provided by Eli Lilly so that they could be blackmailed into prescribing Lilly’s drugs. For those interested, this is a brief talk that Virapen gave about his experiences. I greatly appreciate the fact he used candid language rather than the euphemisms almost everyone else does:
At the start of the saga, Lilly’s senior management knew Prozac was garbage and wanted to shelve the drug, but since Lilly in dire financial straits they decided to go all in on the approval of Prozac in the hope it could save the company. Prozac, in turn, had initially been proposed as a treatment for weight loss (as this side effect of Prozac had been observed in treatment subjects).
However, Lilly ultimately concluded (as explained above) it would be much easier to create the illusion Prozac treated “depression” and then get a post-marketing approval for the treatment of weight loss.
As Prozac took off, it became clear that depression was a much better market, and the obesity aspect was forgotten. Lilly then used a common industry tactic and worked tirelessly to expand the definition of depression so that everyone could become eligible for the drug and aggressively marketed this need for happiness to the public, before long, transforming depression from a rare to a common one.
Unfortunately, while the marketing machine had no difficulties creating a demand for Prozac, the initial clinical trial data made it abundantly clear that the first SSRI, Prozac, was dangerous and ineffective. Lilly settled on the strategy of obtaining regulatory approval in Sweden, and using this approval as a precedent to obtain approval in other countries.
Virapen was assigned to this task and told by his superiors that if he failed, his career was over. Virapen, unfortunately, discovered that whenever he provided Lilly’s clinical trial data to experts, they laughed and had trouble believing he was actually seeking regulatory approval as Prozac’s trial data was just that bad.
Sweden (following their regulatory procedures) elected to allow an outside independent expert to make the final determination on whether Prozac should be approved or not. The identity of this expert witness was concealed, but Virapen was able to determine that it was Anders Forsman, a forensic psychiatrist and member of the legal council on the Swedish National Board of Health.
After meeting with Virapen, Forsman proposed an untraceable bribe. Then, upon receiving payment, wrote a glowing letter in support of Prozac, fully reversing his previous position (he had ridiculed it just two weeks before) and guided Virapen through re-writing the trial to conceal the 5 attempted (4 of which were successful) SSRI suicides in it.
Forsman’s “expert” opinion resulted in Prozac being partially approved and formally priced for reimbursement in Sweden, which was then used as a precedent to market it around the world at that same lucrative price.
Note: After leaving Lilly, Virapen tried to have Forsman prosecuted for bribery. Despite the chairman for the Institute against Bribery submitting a report to the Department of Justice affirming bribery had indeed occurred, Forsman (who repeatedly lied throughout the process) was not prosecuted because he was not an official employee of the agency. Forsman in turn was allowed to continue his professional career and was employed by the state long after the investigation ended.
Virapen noted that during this time, German drug regulators who had clearly and unambiguously stated that Prozac was “totally unsuitable for the treatment of depression” suddenly reversed their position, leading Virapen to suspect that similar under-the-table activity must have occurred in Germany.
David Healey, a doctor and director of the North Wales School of psychological medicine, likewise concluded that the German approval was due to “unorthodox lobbying methods exercised on independent members of the regulatory authorities.”
Note: A key reason why the German regulators initially refused to approve Prozac was because the specific criteria used for determining an improvement in depression was highly subjective and the benefit was only being reported by the trial psychiatrists but not the participants themselves.
Not long after saving Eli Lilly, Virapen was fired. Virapen believes he was fired because he was a man of color in an otherwise Caucasian company (he was told this by his supervisor).
Peter Gøtzsche, a leading expert in pharmaceutical research fraud, on the other hand, attributed this to typical organized crime tactics where Lilly sought to conceal their illegal activity by firing Virapen and his two assistants (as immediately after their abrupt termination, none of them were permitted to access their offices, and thus could not obtain any of the files that proved that they had bribed Forsman).
In short, given how horrendous the data supporting their safety and efficacy was, you must be wondering how the SSRIs made it through the regulatory approval process.
George H.W. Bush
There is a lot of dark history to the Bush family. The Bush dynasty was founded by Prescott Bush, who built his family fortune by collaborating with the Nazis directly against the wishes of the U.S. government (The Guardian, for example, confirms it here).
His son, George H.W. Bush had the unique accomplishment of being the only CIA chief to later become president, and during his brief tenure there was responsible for numerous crimes against humanity in South America. After leaving the CIA once Carter became president, Bush (senior) served as a board member for Eli Lilly.
He then joined the Reagan Administration as Vice President, where he helped to push through the catastrophic decision for the FDA to approve aspartame for consumer use (aspartame was so dangerous even the FDA did not want to approve it). After succeeding Ronald Reagan as President, Bush chose Dan Quayle as his Vice President:
“In Talking Back to Prozac (1994), I pointed out that Prozac was approved under the first Bush administration and that George Bush had been a member of the board of directors of Eli Lilly, the manufacturer of Prozac. I also pointed out that Vice President Dan Quayle was from Indiana, the home state and international headquarters for Eli Lilly.
At the time the FDA was approving Prozac, Quayle employed former Eli Lilly personnel on his own staff, and Quayle had considerable leverage over the FDA as the chair of a special committee that was investigating its operations.
I questioned whether the FDA might have rejected Prozac and that the entire SSRI onslaught might never have gotten started if the president and vice president of the United States had not been so closely affiliated with Eli Lilly.”
Bush’s son, President George W. Bush likewise followed in his father’s footsteps and appointed Eli Lilly executives to senior positions within his administration. In fact, he even inserted a provision into the Patriot Act to exempt vaccine manufacturers, including Eli Lilly, from liability for thimerosal (Mercury) within vaccinations.
In short, Bush profoundly changed the FDA’s regulatory conduct. Consider this example shared by John Virapen that occurred a few years before Bush became president. In 1980, Eli Lilly applied for the approval of benoxaprofen, and aggressively promoted this new blockbuster medication.
Not long after being approved, in 1982, benoxaprofen was taken off the market after being linked to a small number of deaths, and Eli Lilly underwent a lengthy investigation conducted by the Justice Department, where it was concluded that Lilly intentionally covered up the deaths caused by their drug. Benoxaprofen is banned, but nothing remotely similar has been done for the SSRIs.

Download this Article Before it Disappears
SSRIs and the FDA
The FDA’s treatment of the SSRIs is one of the only instances I know of, where, like the COVID vaccines, the agency has not only ignored, but actively tried to conceal a horrific number of adverse events for a pharmaceutical despite receiving widespread protest from the public. This was most likely heavily influenced by the Bush Administration being in bed with Eli Lilly.
As such, it is insightful to see how this has played out over decades, as we ponder how the FDA will handle the COVID vaccines and what we need to do to address this mess. First, consider the FDA’s behavior when Bush was not yet the president:
“Initially, the FDA was skeptical and noted serious flaws in Lilly’s trials. An FDA officer wrote in 1984 that patients who didn’t do well after two weeks had their blinding broken, and if they were on placebo, they were switched to fluoxetine (resulting in six weeks of fluoxetine being compared to two weeks on placebo).
An FDA review also discovered that 25% of the patients had taken an additional drug, and when the FDA in 1985 removed patients on other drugs from Lilly’s trials, there was no significant effect of fluoxetine.
By adding benzodiazepines, Lilly broke the rules for its trials but didn’t inform the FDA, and when the FDA later learned about it, the agency permitted it and thereby broke its own rules. The public and the doctors were never informed about this ruse.”
Prozac was ultimately approved in December 1987, at which point 3 of the 4 studies that this approval was based upon used benzodiazepines to conceal the agitating or psychotic syndromes created by the SSRI drugs.
Note: A good case can be made that many of the benefits attributed to SSRIs actually were due to the benzodiazepines that were used concurrently with them.
Once Prozac entered the market in 1988, adverse event reports began to accumulate, and by 1991, Prozac had one of the highest rates of adverse events ever reported to FAERS (similar to VAERS but for other pharmaceutical injuries).
As there was less regulatory capture at the time, these red flags were sufficient to convene a Congressional hearing on the SSRIs (whereas today, except for one held a month ago by Congresswoman Marjorie Taylor Greene, this still has not happened for the COVID-19 vaccines).
Note: In the first nine years, the FDA received 39,000 adverse event reports, far more than for any other drug. In those, there were thousands of suicides (e.g., by 1999 over 2000 Prozac suicides had been reported), horrendous crimes, hostility, psychoses, confusion, abnormal thinking, convulsions, amnesia and sexual dysfunction.
A 1991 FDA hearing was convened where many witnesses told stories about out-of-character suicides and homicides. The advisory committee members, many of whom had financial ties to pharmaceutical companies producing SSRIs, ignored those reports and unanimously rejected the following proposal:
“There is credible evidence to support a conclusion that antidepressant drugs cause the emergence and/or the intensification of suicidality and/or other violent behaviors.”
Note: Internal Lilly documents revealed that the FDA had already been working with Lilly on the suicide issue (and that previously Lilly had disclosed to German regulators that Prozac doubled the risk of suicide compared to placebo). However, at the meeting, the chair of the FDA committee interrupted an outside expert who tried to share this, resulting in most of the presentation being conducted by Lilly employees who were able to present Lilly’s narrative to everyone).
Similarly, at the time this hearing occurred, the FDA’s own employees had been raising concerns about the safety of Prozac. Furthermore, a later obtained document showed that the FDA knew that the suicide rate on Prozac was 0.52% (vs. 0.18% on placebo), and that in Pfizer’s Zoloft submission (which reported a 26% decrease in suicide attempts), when the FDA counted the deaths correctly, there was actually a 29% increase in them.
Sadly, buying out “expert” committees is a standard industry practice. To further illustrate the illegitimacy of these committees (who are entrusted to decide much of public policy), consider this report from Kim Witczak, a citizen activist who was able be appointed to one of them:
“Fast forward, after Pfizer settled the Chantix lawsuits Pfizer went to the FDA to ask to have the black box neuropsychiatric warning removed from their drug label. By this time, I was the Consumer Representative on the FDA Psychopharmacologic Drugs Advisory Committee.
We were going to review Pfizer’s new EAGLE study. I was really looking forward to being part of this committee and had many questions to ask about the safety, the lawsuits, the internal company documents discovered and reviewed by experts, and most importantly, the victims.
After all, Pfizer just settled the lawsuits for almost $300 million and silenced everyone. One would think the FDA committee would want to have all information including what was discovered in lawsuits involving 2700+ victims before making any decisions to remove the warnings.
A few days before the FDA Advisory Committee, I received an email from the FDA that they wanted to talk with me about the upcoming advisory committee meeting. Someone (cough Pfizer) brought it to their attention that I had an “intellectual bias” and shouldn’t serve on the committee.
The roomful of FDA staffers told me that I was being recused from serving on this meeting. I told them if they think safety is an intellectual bias (or a point of view), I will always have one.
Much to their surprise, I said I would still like to address the committee and speak during the open public hearing. I ended up flying out a few days later on my own time and dime to make sure my comments and questions were asked even though they wouldn’t be part of the official public record of this meeting.
Ultimately, in an unprecedented move, the FDA removed this serious black box warning that involved violence, hallucinations, suicide, and other psychiatric side effects. To this day, this story has never really been told by the media. These side effects didn’t suddenly go away. Just the FDA black box warnings.”
As detailed above, lawsuits against SSRI manufacturers like Lilly have repeatedly revealed those companies deliberately concealed the adverse events that occurred in their trials. Similarly, Lilly also chose to commit fraud by illegally failing to report 76 of 97 cases of suicidality from Prozac in a post-marketing surveillance study it submitted to the FDA.
Furthermore, Lilly also failed to report that, Cymbalta, an SNRI frequently marketed for treating chronic pain, was found to cause severe withdrawals once discontinued in half of those who had received it for at least 8 weeks. In turn, in the first quarter of 2012, more reports were submitted to the FDA on serious drug withdrawal effects for Cymbalta than for any other regularly monitored drug, including two opioids.
Note: Paxil is also notorious for being highly addictive (e.g., in their original license application they stated 30% of trial subjects experienced withdrawals), but for the first ten years it was on the market, GSK adamantly claimed it was not addictive. Eventually (in 2001) the WHO stated Paxil had the greatest withdrawal issues of any SSRI on the market (which was followed by a warning from the FDA in 2002).
GSK in turn finally “admitted” this by revising its prescribing instructions to state the risk of withdrawals was not 0.2% but instead 25% (a 125 fold increase).
Organized Cover-Ups
One of the most blatant examples of how far the FDA will go to protect the industry occurred in 2003, when while examining a clinical trial for giving Paxil to children, the FDA noticed that more episodes of “emotional lability” (rapid, often exaggerated changes in mood) were reported in children on Paxil than those on a placebo.
The FDA decided to investigate what the actual symptom Paxil’s manufacturer was concealing behind this label, and was informed most cases referred to suicidality. One of the FDA’s safety officers, Andrew Mosholder, a child psychiatrist, further investigated this issue and concluded that 22 studies showed that children given antidepressants were nearly twice as likely to become suicidal as those given placebos.
His superiors at the FDA who had recently hidden Paxil’s tendency to cause suicidality in children predictably disputed his report, and did not allow it to be released to the public or presented at an advisory meeting. A year later in 2004, the report was leaked, and in a very telling move, the FDA chose to conduct a criminal investigation of the leak rather than address the clear safety concerns it had raised.
Kim Witczak spearheaded many different initiatives against the SSRIs. For example, she filed a wrongful death, failure to warn lawsuit against Pfizer (which Pfizer responded to by sending investigators around her neighborhood to dig up dirt on her). Her lawsuit was able to obtain many crucial documents from Pfizer proving that they knew how dangerous their SSRI was (including the same out-of-body experiences which her husband had had before killing himself).
Her lawsuit eventually provided the ammunition to get a black box warning (easily visible red-alerts the FDA occasionally mandates for pharmaceuticals) placed on the SSRIs.
Note: Documents showed that Lilly initially planned to have a warning for Prozac causing psychosis in the USA package insert, but ultimately only did so in Germany, as their regulators, unlike the FDA, required Lilly to insert this warning.
Because of her efforts, like the previous example showed, Witczak was provided with a direct view into the corruption within the FDA. For example, this is how they addressed the “problem” that lawsuits against the SSRI manufacturers were causing their confidential documents (detailing the actual harms of the drugs) to be released:
“Pfizer used the FDA to intervene in Baum Hedlund’s civil lawsuits. It was discovered that Pfizer paid industry defense lawyer Dan Troy $300k for some legal work shortly before he was appointed FDA Chief Counsel by President Bush. In his new role at the FDA, Dan Troy was the mastermind behind the FDA preemption amicus “friend of the court” brief intervening on behalf of pharmaceutical companies in civil lawsuits.
The brief [falsely] argued that because drug was FDA approved, the lawsuits were “preempted” and should be dismissed.
The brief [falsely] claimed even if a company wanted to warn consumers, the FDA wouldn’t let them update their warning label if the FDA didn’t agree. Many Zoloft suicide lawsuits were tossed out by judges who believed the FDA was final authority on the drug label. Pfizer even tried arguing the FDA preemption brief in my lawsuit. Not once, but twice.
Federal Chief Justice James Rosenbaum disagreed with Pfizer and allowed my lawsuit to proceed.
We worked with NY Representative Maurice Hinchey to help expose the $300k Dan Troy received from Pfizer. Ultimately Dan Troy resigned his FDA Chief Counsel post but not before damage was done. He ultimately went back to work for private industry including becoming global Chief Counsel at GlaxoSmithKline, the maker of Paxil, another SSRI.”
Sadly, paying off regulators (e.g., by giving them cushy jobs of the pharmaceutical industry) is very common (the practice is known as the “revolving door”). For instance, many of the authors of government studies (e.g., FDA employees) who questionably determined the SSRIs were “safe and effective” were also paid off by the SSRI manufacturers.
In 2004, due to the mounting political pressure, the FDA finally released a black box warning linking SSRIs to increased suicidality in children. Despite knowing about this problem long before the SSRIs came to market, it took over two decades for the FDA to provide this critical warning.
More importantly, this only happened after massive public pressure, countless lawsuits proving these effects were deliberately concealed by the manufacturers, public hearings, and leaked reports publicly shaming the FDA.
Note: In 2006, the warning was extended to everyone under the age of 25. As this cut off was completely arbitrary (many of the SSRI suicides occurred in much older individuals) a large press conference was organized the day beforehand so those believing it needed to be applied to all ages could have the time to speak the FDA would not permit them to have during its hearing.
Although their action did not convince the FDA to change course, next year in 2006, the FDA did and applied that warning to all ages groups.
By 1990, the public was demanding for the FDA to determine if SSRIs were linked to increased suicidality. As the evidence proving this was unambiguous, the FDA deliberately avoided publishing a report on this topic. Sixteen years later, shortly after the FDA was exposed for suppressing the link between suicidality in children and SSRIs, the FDA finally published a meta-analysis addressing this question.
The 2006 meta-analysis encompassed 372 placebo-controlled trials of SSRIs (and related drugs) involving 100,000 patients, and showed that up to the age of 40, SSRIs increased suicidal behavior, while in older patients SSRIs decreased this risk.
Note: A common tactic in the pharmaceutical industry is to hyper-focus on one specific set of side effects so that the other side effects can be covered up.
For example, from comparing the incidences of blood clots I hear about relative to the percentage of people who chose the J&J vaccine, I am relatively certain that the mRNA vaccines are more likely to cause blood clots than J&J’s, but whenever this topic is raised, people default to believing only J&J can cause blood clots since it was linked to a few cases of central venous thrombosis and there was a brief period where the vaccine was suspended by the FDA to “assess” this risk.
I suspect that the FDA’s long-delayed meta-analysis and the black box warning were a direct response to the leaked report proving an indisputable link between SSRIs and adolescent suicidality that was produced to shield the other side effects from scrutiny. Sadly, these warnings have done very little to curb the usage of these drugs, as evidenced by how large their market has become.
Rather they served as a way to protect that market as they both were an alternative to pulling the drugs (which is what should have happened) and downplayed the side effects as much as possible (e.g., borrowing from the industry’s playbook, “abnormal thoughts” became abnormal dreams).
Furthermore, the FDA’s meta-analysis almost certainly also understated the risk. For example, the FDA gave the studies they analyzed a free pass on the variety of design flaws that made it easy to conceal their adverse events. In fact, the FDA reached out to many of the SSRI manufacturers and asked them to adjudicate (remove) possibly suicide-related adverse events in their trials as they saw fit and send those results to the FDA.
When analyzing the 2006 meta-analysis, Gøtzsche found numerous other signs of deliberate fraud by the FDA. For example, in many cases (often due to data revealed from litigation), a single study within the meta-analysis was shown to contain more cases of suicide from an SSRI than the 5 suicides the FDA claimed had occurred throughout all 372 of its studies.
From extensively reviewing all the data, Peter Gøtzsche, reached the overall conclusion that there are likely to have been 15 times more suicides on antidepressant drugs than reported by the FDA in its 2006 meta-analysis.
Note: In 2006, 35 million was spent by American’s National Institutes of Mental Health to conduct the STAR*D study, which assessed if SSRIs cured “treatment resistant” depression (making it the largest study on SSRI efficacy ever conducted) and was designed to assess typical patients in real life scenarios (although the care they received was likely better than what is seen in clinical practice).
It found 3% or less of subjects had their depression cured (with it not remitting for the year of observation within the trial). However, the NIMH repeatedly stated “about 70% of those who did not withdraw from the study became symptom-free,” significantly exaggerated the improvements in the patients, and that SSRI treatment was far more effective that placebo, despite no placebos being used in the trial.
In my personal opinion, when your results are off by an order of magnitude, this can only occur through deliberate fraud, something many of us have regrettably come to realize has occurred at both the CDC and the FDA throughout the COVID-19 vaccination campaign.
As it so happened, by 2013, the FDA employee in charge of the 2006 meta-analysis had completely transitioned to the private sector and had made a consulting firm dedicated to helping psychiatric drugs sail through the FDA.
Note: A variety of other large studies have used similar methods to conceal the dangers of the SSRIs. Since I can’t cover all of them here, I chose to focus on ones conducted by the US government.
The Big Lie
When Hitler wrote Mein Kampf in 1925, he described how people could be induced to believe a colossal a lie because they would not believe that someone “could have the impudence to distort the truth so infamously.” While he initially used this idea to attack others (e.g., the Jews), before long he fully adopted it, allowing the Nazi regime to become one of the most powerful forces of propaganda in history.
Many others have also used this approach. For example (as discussed in a recent article), for decades, US health authorities (and professional medical associations) have repeated the mantra that their vaccine is “safe and effective” while simultaneously suppressing all evidence to the contrary (e.g., from their own scientists).
This in turn has resulted in numerous disastrous vaccines (which everyone knew were bad) being pushed onto the market and not being taken off until a significant amount of injuries had occurred. With the SSRIs, we see a similar degree of audacity, as time and time again the SSRI advocates will insist their drugs are safe and effective despite all evidence to the contrary. For example:
“In 2014, the medical director at the Norwegian drug agency, Steinar Madsen, said at a meeting that antidepressants work for 50-60% of the patients. I [Peter Gøtzche] replied that his statement illustrated why we cannot trust our drug regulators and reminded him that the FDA had found in their analysis of 100,000 patients that antidepressants worked for only 10% of the patients.
Throughout the 1990s, while swearing publicly that fluoxetine didn’t increase the risk of suicide or violence, Lilly quietly settled lawsuits out of court and kept the incriminating evidence hidden by obtaining court orders to seal the documents.
[In 2011 the CEO of a company that sold five antidepressants], claimed in a radio programme that SSRIs reduce suicides in children and adolescents. When the stunned reporter asked him why the package inserts warned against suicide attempts, also for Lundbeck’s drugs, he replied that he expected the leaflets would be changed by the authorities!
The radio interview took place while Lundbeck’s US partner, Forest Laboratories, was negotiating compensation with 54 families whose children had committed or attempted suicide under the influence of Lundbeck’s antidepressant drugs.
[BBC Journalist] Shelley Joffre, showed that the GSK spokesperson, Dr Alastair Benbow, lied in front of a running camera. He denied, for example, that paroxetine could cause suicidality or self-harm while he sent data to the drug regulator one month later that showed exactly this, and which immediately led to a ban on using paroxetine in children.”
Note: The UK drug regulators also lied to the public to cover for GSK (which is based in the UK) by stating that the discovery Paxil caused those suicides was completely new to the company (whereas documents showed it had in fact known about it for at least eight years). Furthermore, when US senator Charles Grassley later asked GSK for how long the company had known that paroxetine increases the suicide risk, GSK repeated this lie, claiming GSK had not detected the risk until 2006.
Given their willingness to blatantly lie, even to a US Senator, it should come as no surprise these companies concocted elaborate ways to silence their critics. For example, GSK has publicly stated:
“Major depressive disorder is a potentially very serious illness associated with substantial morbidity, mortality, suicidal ideation, suicide attempts and completed suicide. Unwarranted conclusions about the use and risk of antidepressants, including paroxetine, do a disservice to patients and physicians.”
Many psychiatrists (especially those being paid off by the pharmaceutical industry) in turn have used similar arguments to silence all criticisms of their drugs. Sadly these tactics are not unique to the psychiatric industry. For example, in a previous article I discussed the significant dangers (and complete lack of benefit) from statins.
In turn, whenever statins are questioned, rather that defend them, cardiologists will often insist you are “killing patients” by scaring them away from the drugs, and this argument has been successfully against both physicians and news programs which questioned statins. In turn, as you might guess, that tactic has also been used against critics of the SSRIs.
“In New Zealand, psychiatrists and suicidologists managed to convince the government [with very weak evidence] that publishing information on suicides causes copycat suicide, which in turn made it a criminal offense for victims or the media to publicly discuss SSRI suicides.”
Likewise, this same playbook has been used against critics of a controversial vaccine. Sadly, since there had been numerous trial runs with other deadly products, by the time COVID-19 happened, the “dangerous misinformation” playbook had been developed, and that label was immediately plastered onto anyone who questioned any part of the pandemic response (e.g., the lockdowns, the suppression of early treatment or the COVID-19 vaccines).
This in turn set the stage for where it somehow became acceptable to argue people should be forced to vaccinate against their will despite a significant amount of evidence (and public opinion) existing that argued against vaccinating. In many ways, this is not that different from how psychiatric medication mandates are often pushed upon patients who (due to their side effects) simply do not want them.
Note: There are many sad stories of this — including numerous ones where the courts supported the psychiatric mandate no matter how much work was done to overturn them.
Conclusion
In my eyes, one of the most important things to consider in this article is just how many people are taking SSRIs, and by extension, just how many injuries the percentages I provided in this article translate to. Whenever a drug is being considered for approval, one of the primary concerns by the regulator used to be the total expected harms suggested by the preliminary data — yet as we can see both in the SSRI saga and throughout COVID-19, that principle has simply been discarded.
As I ponder how things could have gotten this way and how symbiotic the relationship has become between the pharmaceutical companies and the drug regulators, I am reminded of this iconic scene from Idiocracy:
The saddest thing about the SSRI saga is that as inexcusable as it was, things were much less corrupt then than they are now, especially within the federal government. At the time that the public challenged the SSRIs, the media would air stories critical of the malfeasance within the federal government and lawsuits could compel the pharmaceutical companies to disclose the harms they were hiding from the public, and Congress was willing to investigate.
Now, all the vaccine manufacturers have almost complete protection from liability and except for a few commentators on Fox News, no one so much as dares to question the vaccines (or any other pharmaceutical for that matter). One comment Kim made on our sad state of affairs really stuck with me:
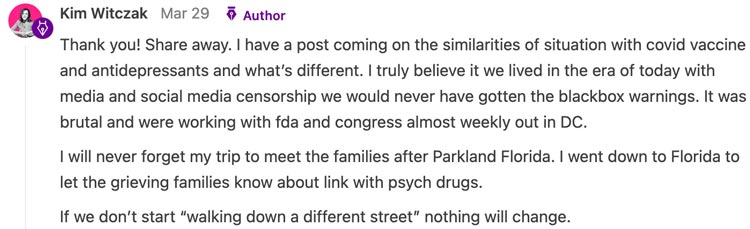
Note: Renowned journalist Sharyl Attkisson has made an excellent case the prolific censorship we have become accustomed to began during the Obama presidency.
My hope is that the harm of the COVID-19 vaccines is so egregious and unambiguous, and more importantly, has affected so many people, that it will prompt enough public outcry to fix or at least improve this systemic corruption.
In this series, I have tried to illustrate how the gross malfeasance that allowed the SSRIs to be brought onto the market and kept there despite countless red flags telling the FDA the drugs were not safe. Overcoming the pressure to take these drugs off the market in turn required a lot of money to be behind those drugs.
In the final part of this series, we will explore how the SSRI industry convinced the world everyone needed their (typically worthless) pills (while simultaneously causing many effective SSRI treatments to be dismissed and forgotten). Much of our culture is shaped by the pharmaceutical industry brands diseases and I believe the tactics they use must be recognized so our society stops falling victim to them.
I thank each of you for reading this series and helping bring attention to this tragedy as many people I am close to have been.
A Note From Dr. Mercola About the Author
A Midwestern Doctor (AMD) is a board-certified physician in the Midwest and a longtime reader of Mercola.com. I appreciate his exceptional insight on a wide range of topics and I’m grateful to share them. I also respect his desire to remain anonymous as he is still on the front lines treating patients. To find more of AMD’s work, be sure to check out The Forgotten Side of Medicine on Substack.

















Thanks! Share it with your friends!
Tweet
Share
Pin It
LinkedIn
Google+
Reddit
Tumblr