By AdminM
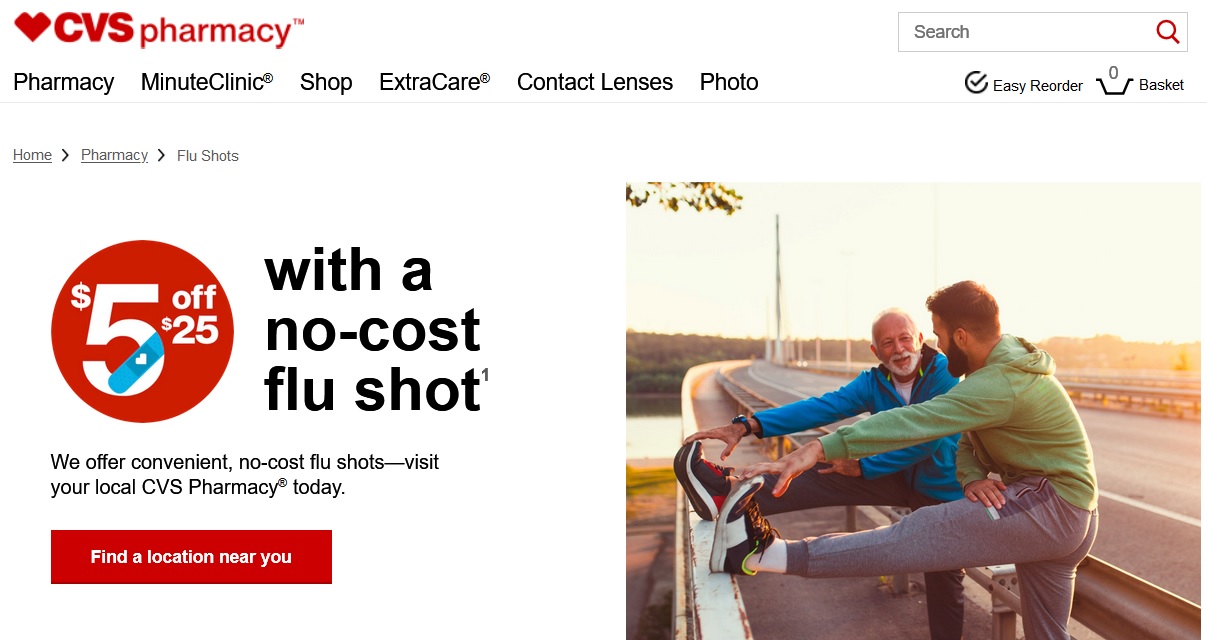
Seniors are frequently targeted for flu shot advertisements. Photo from CVS.com.
by Brian Shilhavy
Editor, Health Impact News
Earlier this month (November, 2019) the FDA approved the Fluzone® High-Dose Quadrivalent influenza vaccine produced by Sanofi Pasteur Inc.
Fluzone is approved for adults 65 years old and above.
Fluzone® High-Dose (Influenza Vaccine) was approved by the FDA in 2009 as a trivalent influenza vaccine, including two influenza A strains and one influenza B strain. Fluzone High-Dose Quadrivalent contains an additional influenza B strain. Fluzone High-Dose Quadrivalent is given to people 65 years of age and older to help prevent influenza disease …read more
Source: Health Impact News
- Rating:
- Views:170 views
- Categories: Health

























Thanks! Share it with your friends!
Tweet
Share
Pin It
LinkedIn
Google+
Reddit
Tumblr